5th Edition of International Neurology Conference 2026, Thailand
- PRECISION GLOBAL CONFERENCE
- huay wang Bangkok
- Thailand
- Neurology, Mental health or psychiatric nursing
5th Edition of International Neurology Conference 2026 huay wang Thailand
Read MoreInternational Summit on Neurology, Neuroscience & Brain Disorders United Arab Emirates
- Aver Conferences
- United Arab Emirates
- Neurology, Mental health or psychiatric nursing
International Summit on Neurology, Neuroscience & Brain Disorders United Arab Emirates
Read MoreInternational Summit on Chemistry & Emerging Technologies United Arab Emirates
- Aver Conferences
- United Arab Emirates
- Public Health
International Summit on Chemistry & Emerging Technologies United Arab Emirates
Read More5th Worldwide Conference on Infectious Diseases London United Kingdom
- Aver Conferences
- London
- United Kingdom
- Infectious Diseases, Public Health, Medical Technology
5th Worldwide Conference on Infectious Diseases London United Kingdom
Read More6th Edition of Euro-Global Conference on Biotechnology and Bioengineering London United Kingdom
- Magnus Group LLC
- London
- United Kingdom
- Healthcare Management
6th Edition of Euro-Global Conference on Biotechnology and Bioengineering London United Kingdom
Read More6th International Conference on Neuroscience and Psychiatry Dubai United Arab Emirates
- Scientex conferences
- Dubai
- United Arab Emirates
- Neurology, Psychiatry
6th International Conference on Neuroscience and Psychiatry Dubai United Arab Emirates
Read MoreInternational conference on Physiotherapy, Medicine and Healthcare 2026 Zurich Switzerland
- Intelli Global Conferences
- Zurich
- Switzerland
- Physiotherapy, Holistic Medicine, Healthcare Management
International conference on Physiotherapy, Medicine and Healthcare 2026 Zurich Switzerland
Read More4th Edition of Global Conference on Gynecology & Women's Health London United Kingdom
- Magnus Group LLC
- London
- United Kingdom
- Gynecology
4th Edition of Global Conference on Gynecology & Women's Health London United Kingdom
Read More4th International Conference on Rare Diseases and Orphan Drugs Barcelona Spain
- Coalesce Research Group
- Barcelona Barcelona
- Spain
- Infectious Diseases
4th International Conference on Rare Diseases and Orphan Drugs Barcelona Spain
Rare Diseases 2026
Read More
Pasadena Tournament of Roses Announces 2026 Rose Bowl Parade Live Streaming Free on Multiple Platforms
Pasadena Tournament of Roses Announces 2026 Rose Bowl Parade Live Streaming Free on Multiple Platforms
Read More2nd Edition of the International Obesity and Metabolism Conference (IOMC 2026) Singapore
2nd Edition of the International Obesity and Metabolism Conference (IOMC 2026) Singapore
2nd Edition of the International Obesity and Metabolism Conference (IOMC 2026)
July 17–19, 2026 | Singapore & Virtual
2nd Edition of Public Health and Midwifery World Conference PHMWC 2026 Miami United States
- PRECISION GLOBAL CONFERENCE
- Miami Florida
- United States
- Public Health, Midwifery
2nd Edition of Public Health and Midwifery World Conference PHMWC 2026 Miami United States
Read MoreInternational Experts Summit on Infectious Diseases (Infectious Summit-2026) Tokyo Japan
International Experts Summit on Infectious Diseases (Infectious Summit-2026) Tokyo Japan
6th International Conference on Public Health, Infectious Diseases & Health Care Research Tokyo Japan
- Inovine
- Tokyo
- Japan
- Public Health
6th International Conference on Public Health, Infectious Diseases & Health Care Research Tokyo Japan
6th International Conference on Public Health, Infectious Diseases & Health Care Research
Dates: October 19–20, 2026
Venue: Tokyo, Japan
Aseptic Processing in the Manufacture of Biotech and Pharmaceutical Products
Aseptic Processing in the Manufacture of Biotech and Pharmaceutical Products
Use coupon COMPLIANCE15 to get 15% discount on this virtual seminar
Read More
Biostatistics for the Non-Statistician
Biostatistics for the Non-Statistician
Use coupon COMPLIANCE15 to get 15% discount on this virtual seminar
Read More
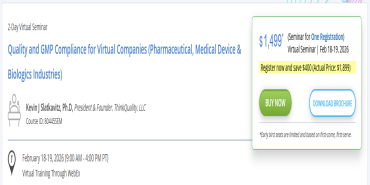
Quality and GMP Compliance for Virtual Companies (Pharmaceutical, Medical Device & Biologics Industries)
Quality and GMP Compliance for Virtual Companies (Pharmaceutical, Medical Device & Biologics Industries)
Use coupon COMPLIANCE15 to get 15% discount on this virtual seminar
Read More
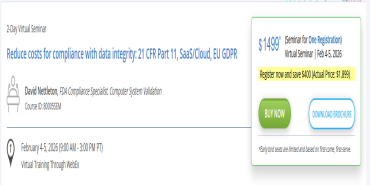
Reduce costs for compliance with data integrity: 21 CFR Part 11, SaaS/Cloud, EU GDPR
Reduce costs for compliance with data integrity: 21 CFR Part 11, SaaS/Cloud, EU GDPR
Read MorePharma Forum 2026: Medical Meetings Mastered Boston United States
- Informa Connect
- Boston Massachusetts
- United States
- Pharmaceutical, Healthcare Management
Pharma Forum 2026: Medical Meetings Mastered Boston United States
Drive excellence in life science meetings and events by fostering bold partnerships, challenging the status quo, amplifying diverse voices, and navigating regulatory complexity with precision.
Read More
WORLD BUSINESS EVENT AND EXHIBITION ON EAR, NOSE AND THROAT CARE Dubai United Arab Emirates
- Utilitarian Conferences Gathering
- Dubai
- United Arab Emirates
- Otorhinolaryngology
WORLD BUSINESS EVENT AND EXHIBITION ON EAR, NOSE AND THROAT CARE Dubai United Arab Emirates
World Business Event & Exhibition on Ear, Nose, and Throat (ENT) Care
Read More6th Dubai International Neurology Congress United Arab Emirates
- Medetarian Conference Organizing
- United Arab Emirates
- Neurology
6th Dubai International Neurology Congress United Arab Emirates
Read More8th Edition of Euro-Global Conference on Pediatrics and Neonatology The Grove, Bath Rd United Kingdom
- Magnus Group LLC
- The Grove, Bath Rd
- United Kingdom
- Pediatrics
8th Edition of Euro-Global Conference on Pediatrics and Neonatology The Grove, Bath Rd United Kingdom
Read More2nd International Conference on Gynecology, Obstetrics and Women's Health 00146 Italy
- C2p Forum
- 00146 Roma
- Italy
- Gynecology
2nd International Conference on Gynecology, Obstetrics and Women's Health 00146 Italy<br/>
Read More10th European Public Health Conference 2026 Helsinki Finland
- Scientific Meditech
- Helsinki
- Finland
- Public Health, Epidemiology
10th European Public Health Conference 2026 Helsinki Finland
Read MoreCopyright © 2015 - 2121 World Medical Events Organization - All Rights Reserved | Terms & Conditions | Privacy Policy